
A temporary COVID-19 testing site can only perform CLIA-waived or FDA-authorized point-of-care tests for SARS-CoV-2 and must be under the direction of the existing laboratory or testing site director.ĬMS has provided specific guidance for the use of FDA authorized OTC self-tests when these tests are either performed or the results are interpreted by someone other than the individual being tested or their parent or guardian. The State Agency should be notified of any changes to the laboratory or testing site ownership, name, address, or director within 30 days.ĭuring the COVID-19 public health emergency, CMS allows a laboratory or testing site to use its existing Certificate of Waiver to operate a temporary COVID-19 testing site in an off-site location, such as a nursing home or drive-through location.
The point-of-care testing site must keep its certificate information current. A non-certified point-of-care testing site will be treated as operating under a Certificate of Waiver while their application is being processed. Laboratories or point-of-care testing sites that have applied for a CLIA Certificate of Waiver to perform SARS-CoV-2 point-of-care testing can begin testing and reporting SARS-CoV-2 results as soon as they have submitted their application to the State Agency, as long as they meet any additional state licensure requirements that apply.
#Rapod covid test how to#
See How to Obtain a CLIA Certificate of Waiver for more information. Pay the CLIA Certificate of Waiver fee, following instructions provided by the State Agency.Send the completed application to the address of the local State Agency for the state where testing will be performed.Complete an application ( Form CMS-116 ), available on the CMS CLIA website or from a local State Agency.A CLIA Certificate of Waiver is appropriate if SARS-CoV-2 point-of-care testing is the only testing being performed. A CLIA certificate is required to perform point-of-care testing. There are four different types of CLIA certificates, any one of which is appropriate for point-of-care testing. Regulatory Requirements for Rapid Testing in Point-of-Care Settings The chance of getting a false positive is low with at-home tests, so following the antigen test directions and CDC guidelines to the letter prevents the possibility of spreading the virus to others.Ĭlick here to read the complete article from Health. That uncertainty is why the CDC recommends five more days of isolation. “Does that mean they're still infectious? We don't know," Vail told. On day six, about 50 percent will still test positive. Centers for Disease Control and Prevention (CDC), there’s still the possibility that someone could test positive.

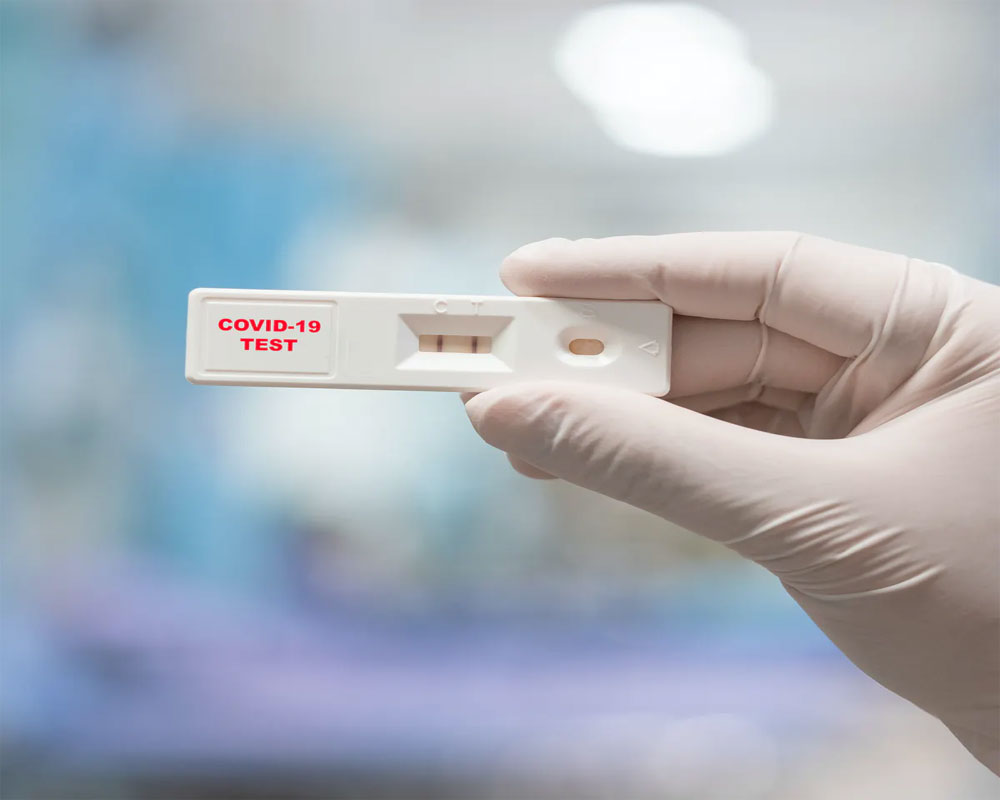
Still, whether the line or lines are bright or faint, two lines still mean positive, one line still means negative, and the safety precautions remain the same: "Five days of and then five days of masking if you have no symptoms,” before going back to your normal routine, Vail said.Īfter following those guidelines from the U.S. A brighter line indicates the patient has more virus in their body and is likely to be sicker and more infectious. A faint line also can mean the tester didn’t swab well enough to provide a good test sample. If the line is fainter, the patient is likely to be less sick, less infectious, or might be nearing the end of infection, Vail said. The opaqueness of the lines also can indicate more, Vail said. If the window on the testing stick shows two lines, the tester is positive for COVID-19. recently interviewed Eric Vail, MD, director of Molecular Pathology, about the at-home antigen test for the virus that causes COVID-19 and how it determines whether the virus is inside the body and, if so, how much virus is there.Īt-home antigen tests work by detecting whether the virus is found in testers’ nasal secretions, Vail told.


 0 kommentar(er)
0 kommentar(er)
